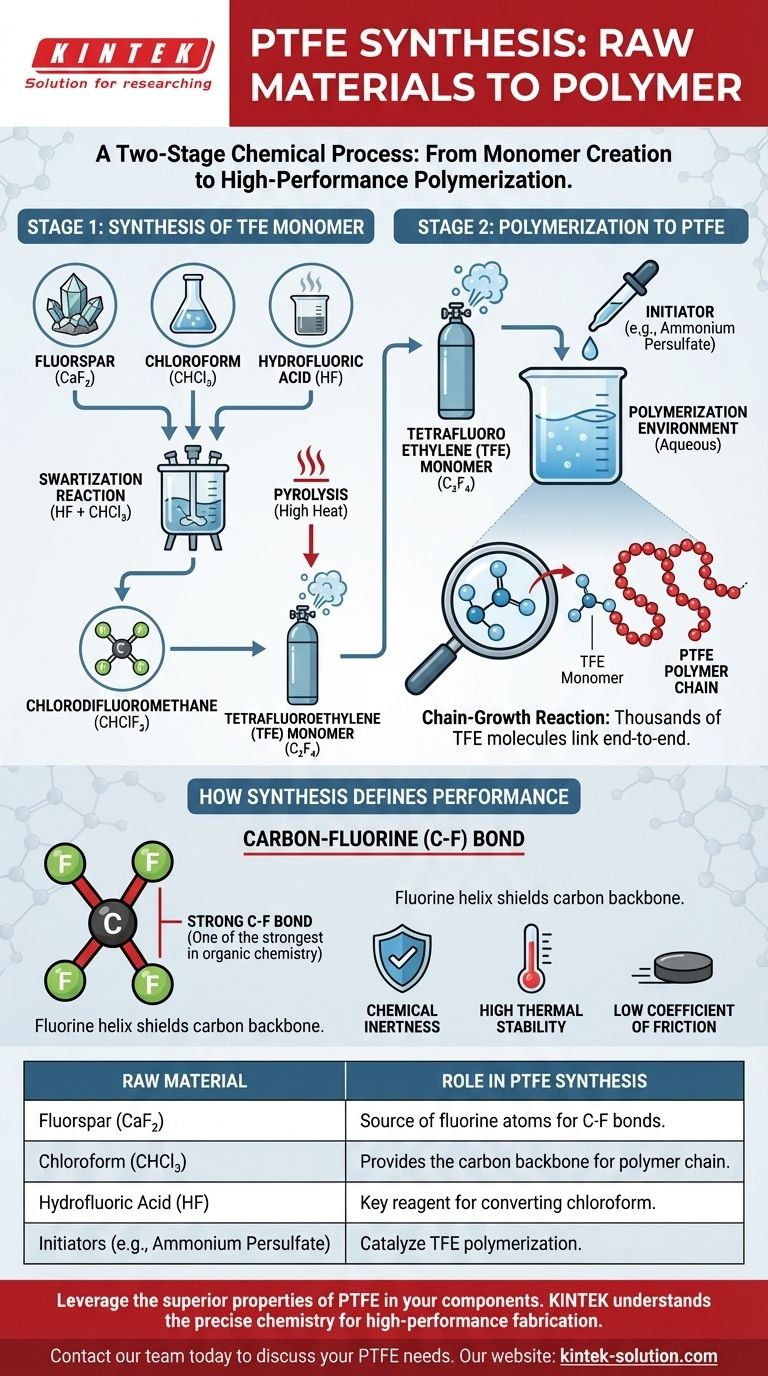
At its core, the synthesis of Polytetrafluoroethylene (PTFE) begins with three primary raw materials: fluorspar, hydrofluoric acid, and chloroform. These foundational components are used to create the tetrafluoroethylene (TFE) monomer, which is the essential building block that is then polymerized into the final PTFE product.
The production of PTFE is a two-stage chemical process. First, common industrial chemicals and a mineral are used to synthesize a highly specific monomer gas (TFE). Second, this gas undergoes a chain-reaction polymerization to form the stable, high-performance polymer.

From Raw Materials to the Core Monomer (TFE)
The journey to create PTFE starts with synthesizing its precursor molecule, tetrafluoroethylene (TFE). This process transforms widely available materials into a specialized fluorocarbon gas.
The Starting Point: Fluorspar and Chloroform
The entire process begins with two key ingredients. Fluorspar, a mineral form of calcium fluoride (CaF2), serves as the essential source for all the fluorine atoms in the final polymer. Chloroform (CHCl3) provides the carbon backbone.
The Key Reaction
These materials are processed to create hydrofluoric acid (HF). The hydrofluoric acid is then reacted with chloroform in a process known as swartization. This reaction produces an intermediate compound, chlorodifluoromethane (CHClF2).
Creating the Monomer Gas
This intermediate compound is then subjected to high heat in a process called pyrolysis. The heat causes the molecules to break down and recombine, forming tetrafluoroethylene (C2F4), or TFE. This TFE is the critical monomer—the individual link that will be chained together to form PTFE.
Polymerizing TFE into PTFE
Once the TFE monomer gas has been synthesized and purified, the final step is to link these individual molecules into the long, stable chains that constitute the PTFE polymer.
The Polymerization Environment
The polymerization of TFE is typically carried out in water. This aqueous environment helps control the reaction temperature and manage the process efficiently.
The Role of Initiators
The reaction does not start on its own. A small amount of a chemical initiator, or catalyst, is required to kick off the polymerization. Common initiators include ammonium persulfate or disuccinic acid peroxide.
The Chain-Growth Reaction
The initiator starts a free-radical chain reaction. A single TFE monomer is activated, which then rapidly bonds to another, and another, in a cascade. This process links thousands of TFE molecules end-to-end, forming the long, high-molecular-weight polymer chains of PTFE.
How Synthesis Defines Performance
The choice of these specific raw materials is directly responsible for PTFE's exceptional properties. Understanding the chemistry clarifies why the material behaves the way it does.
The Strength of the Carbon-Fluorine Bond
Fluorine, sourced from fluorspar, is the most electronegative element. When it bonds with carbon, it creates the carbon-fluorine (C-F) bond, one of the strongest single bonds in organic chemistry.
The Source of PTFE's Inertness
The long PTFE polymer chain is essentially a carbon backbone completely sheathed by a tight helix of fluorine atoms. This stable and powerful C-F bond shield is what gives PTFE its hallmark characteristics: extreme chemical inertness, high thermal stability, and a very low coefficient of friction.
Making the Right Choice for Your Goal
Understanding the synthesis pathway from raw material to final polymer provides critical context for material sourcing and application.
- If your primary focus is supply chain and origin: Recognize that PTFE production is fundamentally tied to the availability of fluorspar, a mined mineral, and foundational industrial chemicals.
- If your primary focus is material performance: Know that the fluorine chemistry, which begins with fluorspar, is directly responsible for the unparalleled thermal and chemical resistance that defines PTFE.
Ultimately, understanding how PTFE is made from these simple inputs demystifies its extraordinary capabilities.
Summary Table:
| Raw Material | Role in PTFE Synthesis |
|---|---|
| Fluorspar (CaF₂) | Source of fluorine atoms for the strong C-F bonds. |
| Chloroform (CHCl₃) | Provides the carbon backbone for the polymer chain. |
| Hydrofluoric Acid (HF) | Key reagent for converting chloroform into the TFE monomer. |
| Initiators (e.g., Ammonium Persulfate) | Catalyze the polymerization reaction of TFE gas into PTFE. |
Leverage the superior properties of PTFE in your components.
At KINTEK, we understand the precise chemistry behind PTFE, allowing us to fabricate high-performance components that meet the demanding requirements of the semiconductor, medical, laboratory, and industrial sectors. Whether you need custom PTFE seals, liners, or labware—from prototypes to high-volume orders—our expertise ensures precision and reliability.
Contact our team today to discuss your PTFE fabrication needs and discover how we can add value to your projects.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Sleeves and Hollow Rods for Advanced Applications
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
People Also Ask
- What are the key considerations when machining Teflon? Master Precision Machining for Soft Polymers
- What tips can improve Teflon machining results? Master Sharp Tools, Heat Control, and Rigid Support
- Why is CNC machining preferred for Teflon parts over other methods? Unlock Precision & Complex Designs
- What are the best practices for achieving tight tolerances in Teflon (PTFE) machining? Master Precision for Demanding Applications
- How does PTFE contribute to low friction and wear resistance? Achieve Superior Performance with Advanced Materials



















