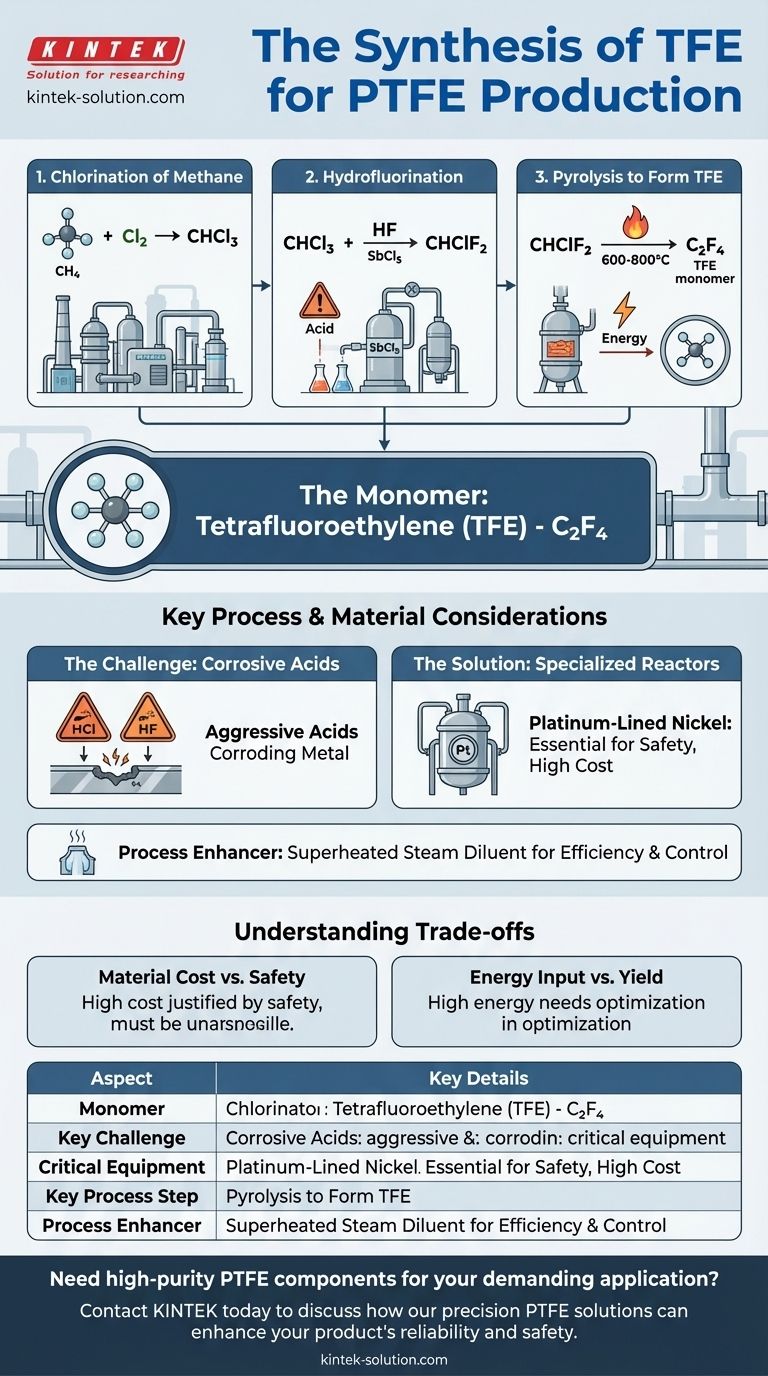
The monomer used in the preparation of PTFE is tetrafluoroethylene (TFE). Its chemical formula is C2F4. The synthesis of this monomer is a chemically aggressive process that demands highly specialized equipment and careful management of reaction conditions to ensure both safety and efficiency.
The core challenge in synthesizing the TFE monomer for PTFE is not the chemistry itself, but managing the extremely corrosive and hazardous materials involved. This requires specialized, corrosion-resistant reactors and carefully controlled process parameters.

The Synthesis Pathway to TFE
The production of tetrafluoroethylene is a multi-step process that starts with common industrial chemicals and escalates in complexity and hazard. The primary goal is to systematically replace hydrogen atoms with fluorine atoms.
Step 1: Chlorination of Methane
The journey begins by reacting methane (CH4) with chlorine (Cl2) to produce chloroform (CHCl3). This is a foundational step in industrial organohalogen chemistry.
Step 2: Hydrofluorination
Next, chloroform is reacted with anhydrous hydrogen fluoride (HF) in the presence of an antimony pentachloride (SbCl5) catalyst. This reaction produces chlorodifluoromethane (CHClF2).
Step 3: Pyrolysis to Form TFE
This is the most critical and energy-intensive stage. Chlorodifluoromethane (CHClF2) is subjected to high-temperature pyrolysis, typically between 600-800°C. This process removes hydrogen and chlorine, causing the molecules to form the highly reactive tetrafluoroethylene (C2F4) monomer.
Key Process and Material Considerations
The synthesis of TFE is defined by the harsh chemical environment it creates. Success depends entirely on managing these conditions effectively.
The Challenge of Corrosive Acids
The process heavily involves two highly corrosive acids: hydrochloric acid (HCl) and hydrogen fluoride (HF). These substances will aggressively attack most standard metals and construction materials.
Requirement for Specialized Reactors
To withstand this chemical attack, the reactors must be constructed from exceptionally resistant materials. Platinum-lined nickel is often used, representing a significant investment and a critical engineering constraint.
Improving Efficiency with Diluents
Controlling the pyrolysis reaction is crucial for maximizing yield and safety. Introducing an inert diluent like superheated steam helps manage the reaction temperature and pressure, improving overall process efficiency and control.
Understanding the Trade-offs
The synthesis of TFE highlights a classic industrial trade-off between material performance and cost.
Material Cost vs. Operational Safety
While materials like platinum-lined nickel are extremely expensive, the cost is justified by the necessity of containing the corrosive reagents. Using inferior materials would lead to rapid equipment failure, process leaks, and severe safety hazards.
Energy Input vs. Monomer Yield
The high temperatures required for pyrolysis consume a significant amount of energy. Optimizing this step involves balancing the energy cost against the desired yield of the TFE monomer, making process control enhancers like superheated steam valuable.
Making the Right Choice for Your Goal
Understanding these factors is crucial for anyone involved in the production or sourcing of fluoropolymers.
- If your primary focus is process safety: The non-negotiable priority is investing in reactors made from appropriate corrosion-resistant materials like platinum-lined nickel.
- If your primary focus is process efficiency: Implementing techniques such as using superheated steam as a diluent is essential for controlling the reaction and maximizing monomer yield.
Ultimately, the successful synthesis of TFE hinges on a deep respect for the aggressive chemistry involved and a commitment to using the correct engineering controls.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Monomer | Tetrafluoroethylene (TFE), C₂F₄ |
| Key Challenge | Managing highly corrosive reagents (HF, HCl) |
| Critical Equipment | Reactors made of specialized materials (e.g., platinum-lined nickel) |
| Key Process Step | High-temperature pyrolysis (600-800°C) of CHClF₂ |
| Process Enhancer | Use of inert diluents like superheated steam for control |
Need high-purity PTFE components for your demanding application?
The complex synthesis of TFE underscores the importance of expert material handling and precision manufacturing. At KINTEK, we leverage deep expertise in fluoropolymer processing to deliver superior PTFE seals, liners, and custom labware.
We serve the semiconductor, medical, and laboratory industries with components that meet the highest standards for purity and performance, from prototypes to high-volume orders.
Contact KINTEK today to discuss how our precision PTFE solutions can enhance your product's reliability and safety.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Bottles for Diverse Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Customizable PTFE Crucibles for Laboratory and Industrial Applications
People Also Ask
- What industrial benefits do PTFE-machined parts offer? Achieve Peak Performance in Demanding Applications
- What are the main applications of PTFE type Teflon? Unlock Its Versatility for Your Industry
- What factors should be considered when choosing between Nylon and PTFE? Select the Right Material for Your Application
- What design considerations are important for custom PTFE parts? Design for Performance & Reliability
- What chemical processing applications involve PTFE-machined parts? Essential Components for Corrosive & High-Purity Systems



















