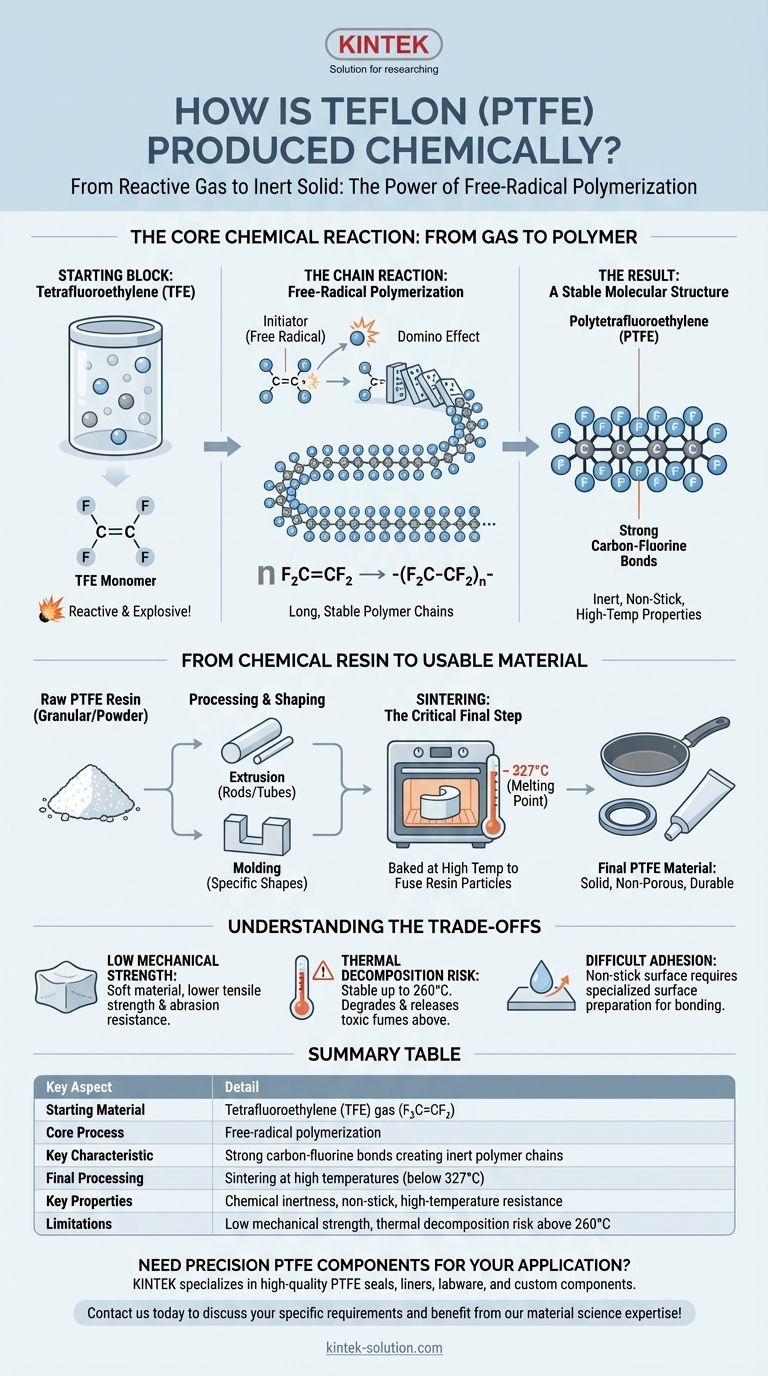
At its core, Teflon (PTFE) is produced through a chemical process called free-radical polymerization. This reaction takes a simple gas, tetrafluoroethylene (TFE), and links its molecules together into extremely long, stable chains. The entire process requires specialized equipment because the TFE monomer can be explosive under certain conditions.
The essence of Teflon production is converting a reactive gas into one of the most inert solids known. This transformation locks highly stable carbon-fluorine bonds into a protective sheath around a polymer backbone, creating the material's signature non-stick, chemical-proof, and high-temperature properties.

The Core Chemical Reaction: From Gas to Polymer
The creation of PTFE is a fascinating example of how a simple starting molecule can be built into a high-performance material. The process hinges on a specific type of chain reaction.
The Starting Block: Tetrafluoroethylene (TFE)
The entire process begins with a single molecule: tetrafluoroethylene, or TFE. Its chemical formula is F₂C=CF₂.
TFE is a colorless, odorless gas. Critically, the double bond between the carbon atoms makes it chemically reactive and ready to form polymers. This reactivity is so high that TFE can decompose explosively, necessitating strict controls during production.
The Chain Reaction: Free-Radical Polymerization
To start the reaction, an initiator (a "free radical") is introduced. This initiator breaks the double bond of one TFE molecule, which then seeks to stabilize itself by bonding with another TFE molecule.
This creates a domino effect. Each newly added molecule extends the chain, rapidly linking thousands of TFE units end-to-end. The chemical equation for this is:
n F₂C=CF₂ → −(F₂C−CF₂)n−
Here, 'n' represents a very large number, indicating a long polymer chain.
The Result: A Stable Molecular Structure
The final product of this reaction is polytetrafluoroethylene—a long chain of carbon atoms forming a stable backbone.
What makes PTFE unique is that this carbon backbone is completely surrounded by a dense, protective sheath of fluorine atoms. The carbon-fluorine bond is one of the strongest known in organic chemistry, and this molecular structure is the source of all of Teflon's famous properties.
From Chemical Resin to Usable Material
The polymerization reaction creates a raw, granular or powdered PTFE resin. To become a useful product, this resin must be consolidated and shaped.
Creating and Processing the Resin
The raw resin produced under high pressure and temperature is the base material for all PTFE products. This resin is then processed into usable forms.
Common methods include extrusion, where the resin is forced through a die to create rods or tubes, or molding, where it is compressed into a specific shape.
Sintering: The Critical Final Step
After being shaped, the PTFE part undergoes a process called sintering. It is baked at a high temperature (but below its melting point of 327°C) to fuse the resin particles together.
This creates a solid, non-porous material with the desired final properties. For coatings on products like cookware, multiple layers of a liquid PTFE dispersion are sprayed onto a prepared surface and then baked to form a durable, bonded finish.
Understanding the Trade-offs
The same chemical structure that provides PTFE's benefits also creates inherent limitations. Understanding these trade-offs is crucial for proper material selection.
Low Mechanical Strength
The weak forces between the individual polymer chains make PTFE a relatively soft material. It has lower tensile strength and abrasion resistance compared to engineering plastics like nylon.
Thermal Decomposition Risk
While PTFE is stable for continuous service up to 260°C (500°F), it begins to degrade at higher temperatures. Heating it above this threshold can release toxic fluorocarbon fumes, which is a critical safety consideration.
Difficult Adhesion
Its famous non-stick property is a double-edged sword. The low surface energy that prevents things from sticking to PTFE also makes it extremely difficult to bond PTFE to other materials using conventional adhesives. Surfaces often must be chemically etched or mechanically roughened to achieve adhesion.
Why This Chemistry Matters for Your Application
Understanding PTFE's production reveals why it behaves the way it does, allowing you to use it effectively.
- If your primary focus is engineering or design: Recognize that the fluorine sheath provides ultimate chemical inertness and lubricity, but its low intermolecular forces result in a soft material unsuitable for high-load structural applications.
- If your primary focus is process safety: Know that the TFE monomer is an explosion hazard and the final PTFE product must never be heated above its service temperature of 260°C (500°F) to avoid producing toxic fumes.
- If your primary focus is manufacturing: Acknowledge that PTFE's non-stick nature requires specialized surface preparation techniques, like grit-blasting or chemical etching, to successfully bond it as a coating.
Ultimately, the carefully controlled polymerization of a simple gas is directly responsible for creating one of the most unique and capable materials in modern industry.
Summary Table:
| Key Aspect | Detail |
|---|---|
| Starting Material | Tetrafluoroethylene (TFE) gas (F₂C=CF₂) |
| Core Process | Free-radical polymerization |
| Key Characteristic | Strong carbon-fluorine bonds creating inert polymer chains |
| Final Processing | Sintering at high temperatures (below 327°C) |
| Key Properties | Chemical inertness, non-stick, high-temperature resistance |
| Limitations | Low mechanical strength, thermal decomposition risk above 260°C |
Need precision PTFE components for your application? KINTEK specializes in manufacturing high-quality PTFE seals, liners, labware, and custom components for semiconductor, medical, laboratory, and industrial applications. Our expertise in precision production and custom fabrication—from prototypes to high-volume orders—ensures you get the exact PTFE solutions your project demands. Contact us today to discuss your specific requirements and benefit from our material science expertise!
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- Why is PTFE approved for medical implants? Leveraging Biocompatibility for Medical Devices
- What are the key characteristics of polytetrafluoroethylene (PTFE)? Unlocking High-Performance Material Properties
- How pure is virgin grade PTFE? The Definitive Guide to Non-Contaminating PTFE
- What is the significance of PTFE's low coefficient of friction? Boost Efficiency & Durability in Your Designs
- What are the characteristics and uses of bronze-filled PTFE? A Guide to High-Strength PTFE Composites
- What is notable about the coefficient of friction of PTFE? Achieve Unmatched Low-Friction Performance
- How does RPTFE differ from PTFE in terms of reactivity? A Guide to Chemical Compatibility
- What is PTFE and what are its notable characteristics? A Guide to Its Unique Properties and Uses



















