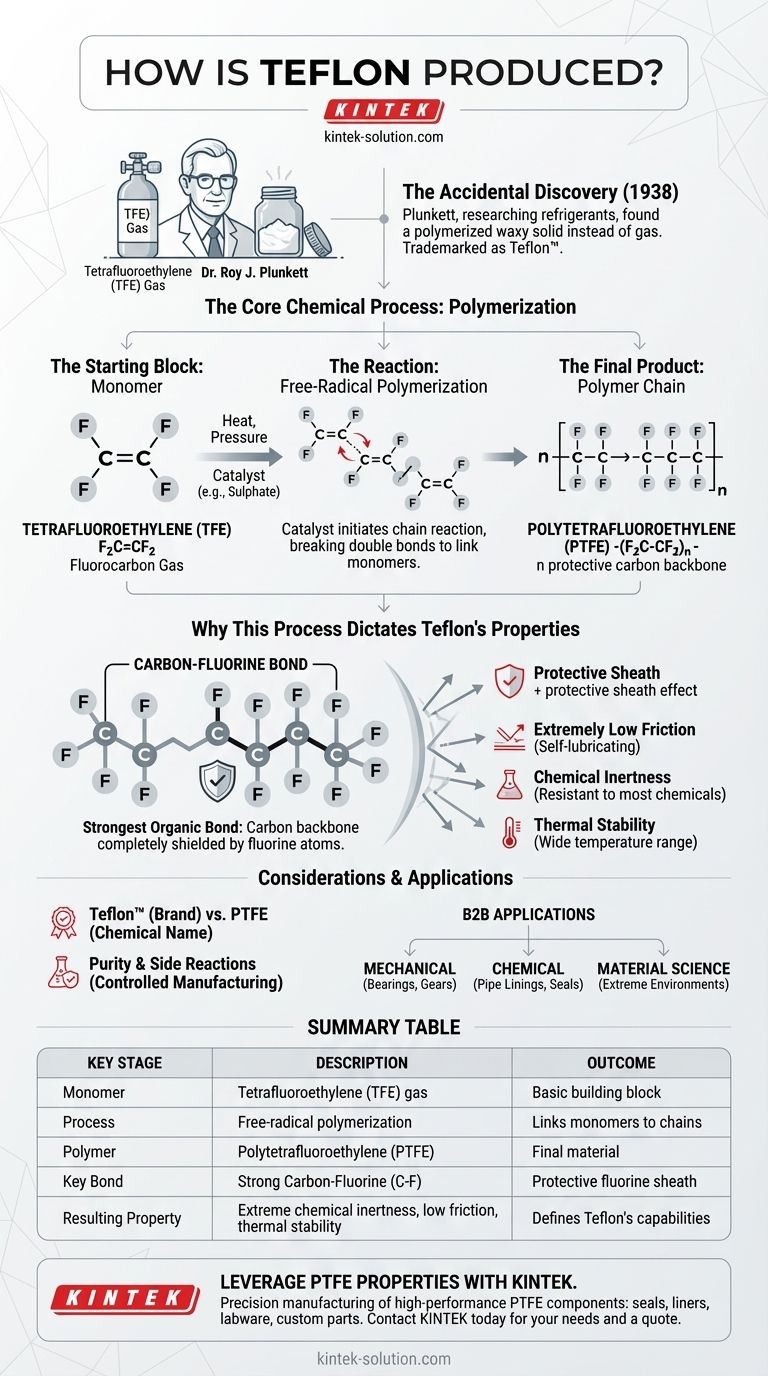
The production of Teflon, chemically known as polytetrafluoroethylene (PTFE), is achieved through a process called free-radical polymerization. This reaction takes the gas tetrafluoroethylene (TFE) and, using a catalyst under high pressure and heat, links thousands of individual TFE molecules together into long, stable polymer chains. The resulting net reaction is n F2C=CF2 → -(F2C-CF2)n-.
The essence of Teflon production is the creation of extremely strong carbon-fluorine bonds, which form a protective "sheath" around a carbon backbone. This simple but powerful molecular structure is the direct source of Teflon's famous non-stick, chemically inert, and temperature-resistant properties.

The Accidental Discovery of a Revolutionary Polymer
A Serendipitous Finding
The discovery of Teflon was entirely unintentional. In 1938, Dr. Roy J. Plunkett, a chemist at DuPont, was researching new, non-toxic refrigerants.
From Gas to a Waxy Solid
Plunkett had stored tetrafluoroethylene gas in a pressure bottle. When he later opened the valve, no gas came out, yet the bottle still weighed as if it were full. Upon cutting the bottle open, he found it was coated with a waxy, white solid that was remarkably slippery and resistant to corrosion. This material was polytetrafluoroethylene, soon trademarked as Teflon.
The Core Chemical Process: Polymerization
The Starting Block: Tetrafluoroethylene (TFE)
The entire process begins with a single, simple molecule called a monomer. For Teflon, this monomer is tetrafluoroethylene (F₂C=CF₂), a fluorocarbon gas.
The Reaction: Free-Radical Polymerization
To create the final material, these individual TFE monomers are joined together in a long chain. This is accomplished through free-radical polymerization.
The process requires high pressure and heat, and it is initiated with a catalyst, such as a sulphate. This catalyst kicks off a chain reaction that breaks the double bonds in the TFE molecules, allowing them to link together end-to-end.
Creating the Polymer Chain
The final product is a polymer: a massive molecule made of repeating structural units. Teflon's structure is -(F₂C-CF₂)n-, where 'n' represents a large number of repeating monomer units. This long-chain structure gives the material its physical strength and flexibility.
Why This Process Dictates Teflon's Properties
The Strength of the Carbon-Fluorine Bond
The connection between a carbon atom and a fluorine atom is one of the strongest single bonds in organic chemistry. Teflon's production process creates a polymer with a backbone of carbon atoms completely surrounded by fluorine atoms.
A Protective Sheath of Fluorine
These fluorine atoms act as a protective sheath around the carbon chain. This sheath is incredibly stable and non-reactive, preventing almost anything from sticking to it or chemically reacting with it. This is the source of both its non-stick quality and its extreme chemical resistance.
Resulting Characteristics
This unique molecular structure, created during polymerization, directly leads to Teflon's key characteristics:
- Extremely Low Friction: Making it self-lubricating.
- Chemical Inertness: It is resistant to nearly all chemicals, except for certain alkali metals.
- Thermal Stability: It can withstand a wide range of high and low temperatures without degrading.
Understanding the Trade-offs and Considerations
The Name: Teflon vs. PTFE
It is critical to distinguish between the chemical and the brand. PTFE is the generic chemical name for the polymer. Teflon™ is the trademarked brand name for PTFE, currently owned by the Chemours Company (a spin-off of DuPont).
Purity and Side Reactions
Controlling the polymerization process is essential. Under certain conditions, the TFE monomer can decompose into other substances, such as tetrafluoromethane and carbon. Manufacturing requires precise control to ensure the purity and integrity of the final PTFE material.
Making the Right Choice for Your Goal
Understanding the origin of PTFE's properties allows you to apply it effectively to specific technical challenges.
- If your primary focus is mechanical engineering: Recognize that its low-friction surface, created by the fluorine sheath, is ideal for self-lubricating parts like bearings, gears, and slide plates.
- If your primary focus is chemical processing: Leverage its extreme inertness, a direct result of the stable carbon-fluorine bonds, for pipe linings, joints, and seals that handle corrosive materials.
- If your primary focus is material science: Appreciate that its thermal stability across a wide temperature range makes it a versatile choice for components exposed to extreme environments.
Ultimately, the polymerization that links simple gas molecules into a fluorine-protected chain is the key to creating one of the most uniquely capable materials in modern industry.
Summary Table:
| Key Stage | Description | Outcome |
|---|---|---|
| Monomer | Tetrafluoroethylene (TFE) gas (F₂C=CF₂) | The basic building block. |
| Process | Free-radical polymerization (heat, pressure, catalyst) | Links monomers into long polymer chains. |
| Polymer | Polytetrafluoroethylene (PTFE): -(F₂C-CF₂)n- | Forms the final PTFE material. |
| Key Bond | Strong Carbon-Fluorine (C-F) bond | Creates a protective fluorine sheath. |
| Resulting Property | Extreme chemical inertness, low friction, thermal stability | Defines Teflon's famous capabilities. |
Leverage the unique properties of PTFE for your most demanding applications.
At KINTEK, we specialize in the precision manufacturing of high-performance PTFE components. Our expertise in custom fabrication—from prototypes to high-volume production—ensures you get the exact seals, liners, labware, and custom parts your industry requires.
Whether you're in the semiconductor, medical, laboratory, or industrial sector, our components deliver unmatched chemical resistance, non-stick performance, and thermal stability.
Contact KINTEB today to discuss your PTFE component needs and get a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- PTFE Chemical Solvent Sampling Spoon
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What are the different types of PTFE and their common uses? Select the Right PTFE for Your Application
- What are the key characteristics of PTFE (Teflon)? Unlock Superior Chemical & Thermal Performance
- What are the features of modified PTFE? Achieve Superior Sealing and Structural Performance
- What are some consumer product applications of PTFE? Discover its Versatility Beyond Non-Stick Pans
- What are some common industrial applications of PTFE? Essential for Extreme Environments
- In which industries is Teflon commonly used? Essential for Chemical, Medical, and Aerospace
- What automotive application benefits from PTFE coating? Enhance Vehicle Reliability and Performance
- What is PTFE chemically composed of? Discover the Simple Chemistry Behind Its Extreme Performance



















