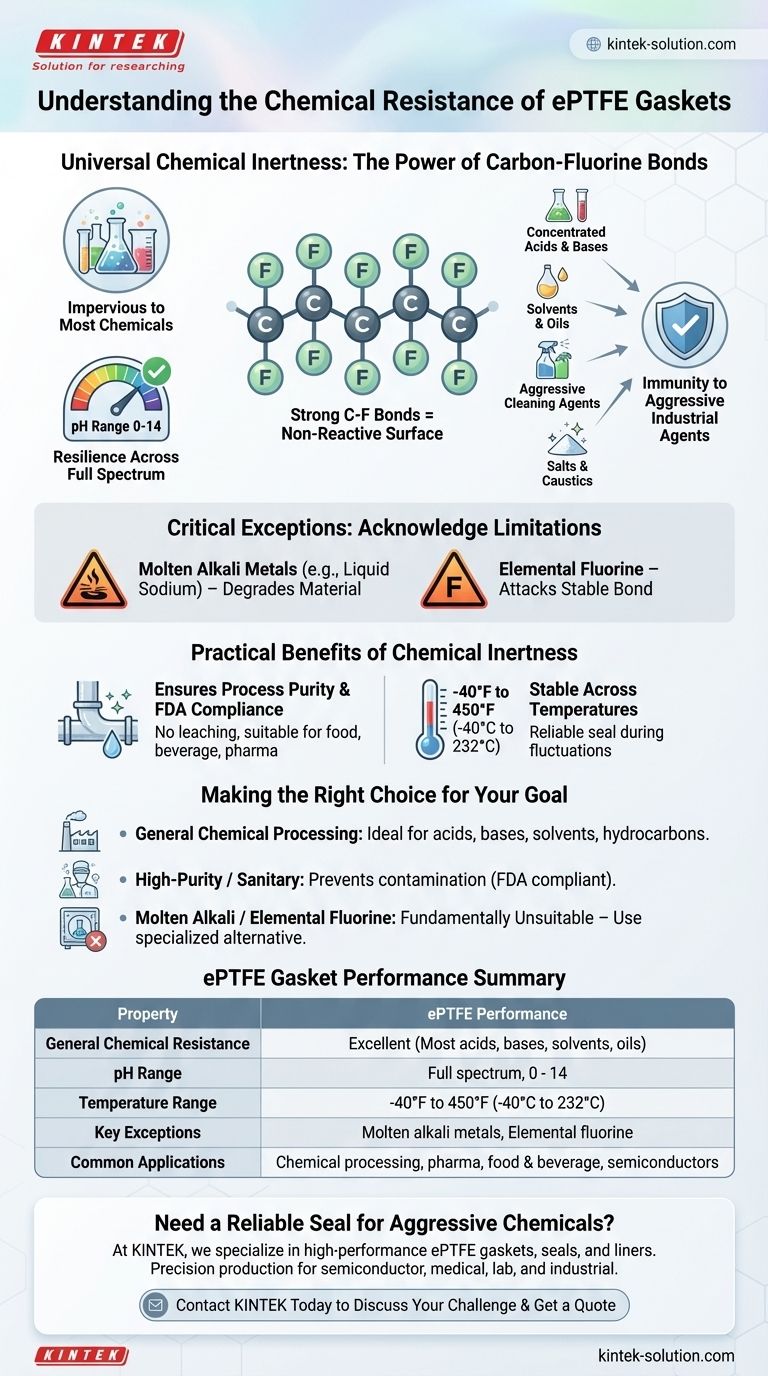
To put it directly, ePTFE (expanded Polytetrafluoroethylene) gaskets offer nearly universal chemical resistance, making them one of the most chemically inert sealing materials available. They are impervious to the vast majority of chemicals across the entire pH spectrum from 0 to 14. The only notable exceptions are highly reactive substances like molten alkali metals and elemental fluorine.
The core reason for ePTFE's exceptional performance is the strength of its carbon-fluorine molecular bonds. This creates a non-reactive surface, ensuring reliability and preventing contamination in almost any industrial chemical application.

The Foundation of ePTFE's Chemical Inertness
The remarkable resistance of ePTFE is not a surface treatment; it is an inherent property of its molecular structure. Understanding this foundation is key to trusting its performance in critical applications.
The Power of the Carbon-Fluorine Bond
PTFE is a fluoropolymer composed entirely of carbon and fluorine atoms. The bond between carbon and fluorine is exceptionally strong and stable, creating a molecule that does not easily react with other chemicals.
Resilience Across the Full pH Spectrum
This molecular stability translates to outstanding performance in both highly acidic and highly alkaline environments. ePTFE maintains its integrity and sealing capability across the entire pH range of 0-14.
Immunity to Aggressive Industrial Agents
In practical terms, ePTFE gaskets are resistant to a wide array of common and aggressive industrial chemicals. This includes:
- Concentrated acids and bases
- Solvents and oils
- Petroleum hydrocarbons
- Aggressive cleaning agents like chlorine dioxide
- Salts and other caustics
Understanding the Critical Exceptions
While ePTFE is close to chemically invincible, no material is perfect. Its two specific weaknesses are found only in extreme and specialized industrial processes. Acknowledging these limitations is crucial for safe and effective material selection.
Molten Alkali Metals
Substances like liquid sodium or potassium are powerful reducing agents. In their molten state, they are reactive enough to physically strip fluorine atoms from the polymer backbone, causing the ePTFE material to degrade.
Elemental Fluorine
Elemental fluorine (free fluorine atoms) is the most electronegative and reactive of all elements. It is one of the few chemicals capable of attacking the highly stable carbon-fluorine bond that gives ePTFE its inert properties.
How This Impacts Your Application
The chemical properties of ePTFE have direct, practical benefits that go beyond simply preventing leaks. Its inertness contributes to process purity, safety, and long-term reliability.
Ensuring Process Purity
Because ePTFE does not react with, stick to, or tarnish when exposed to chemicals, it will not leach contaminants into the process media. This makes it compliant with FDA regulations and ideal for high-purity applications in the food, beverage, and pharmaceutical industries.
Stability Across Temperatures
ePTFE's chemical resistance remains stable across a broad operational temperature range, typically from -40°F to 450°F (-40°C to 232°C). This ensures a reliable seal even when temperature fluctuations could otherwise accelerate chemical reactions.
Making the Right Choice for Your Goal
Ultimately, selecting a gasket is about matching the material to the demands of the environment.
- If your primary focus is general chemical processing: ePTFE is an exceptionally safe and reliable choice for sealing acids, bases, solvents, and hydrocarbons.
- If your primary focus is high-purity or sanitary applications: The inertness and FDA compliance of ePTFE make it an ideal material to prevent process contamination.
- If your process involves molten alkali metals or elemental fluorine: ePTFE is fundamentally unsuitable, and you must select a specialized alternative material designed for these unique conditions.
By understanding both its incredible strengths and its specific limitations, you can deploy ePTFE with confidence in the most demanding sealing environments.
Summary Table:
| Property | ePTFE Gasket Performance |
|---|---|
| General Chemical Resistance | Excellent (Resistant to most acids, bases, solvents, oils) |
| pH Range | Full spectrum, 0 - 14 |
| Temperature Range | -40°F to 450°F (-40°C to 232°C) |
| Key Exceptions | Molten alkali metals, Elemental fluorine |
| Common Applications | Chemical processing, pharmaceuticals, food & beverage, semiconductors |
Need a reliable seal for aggressive chemicals?
At KINTEK, we specialize in manufacturing high-performance ePTFE components, including custom gaskets, seals, and liners. Our precision production ensures a perfect, contaminant-free seal for your most demanding applications in the semiconductor, medical, laboratory, and industrial sectors.
We offer custom fabrication from prototypes to high-volume orders to meet your exact specifications.
Contact KINTEK today to discuss your chemical sealing challenge and get a quote.
Visual Guide
Related Products
- Custom PTFE Parts Manufacturer for Teflon Containers and Components
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Measuring Cylinders for Advanced Scientific and Industrial Applications
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Volumetric Flasks for Advanced Scientific and Industrial Use
People Also Ask
- What is the temperature range that PTFE can withstand? From -200°C to +260°C for Demanding Applications
- Why is dimensional stability a concern when machining PTFE? Ensure Accurate, Stable PTFE Components
- How does PTFE compare to other low-friction plastics like UHMW-PE and Nylon? A Guide to Material Selection
- What are some applications of CNC machined PTFE parts? Critical Components for Medical, Electrical & Food Industries
- When and by whom was PTFE discovered? A Tale of Accidental Innovation



















