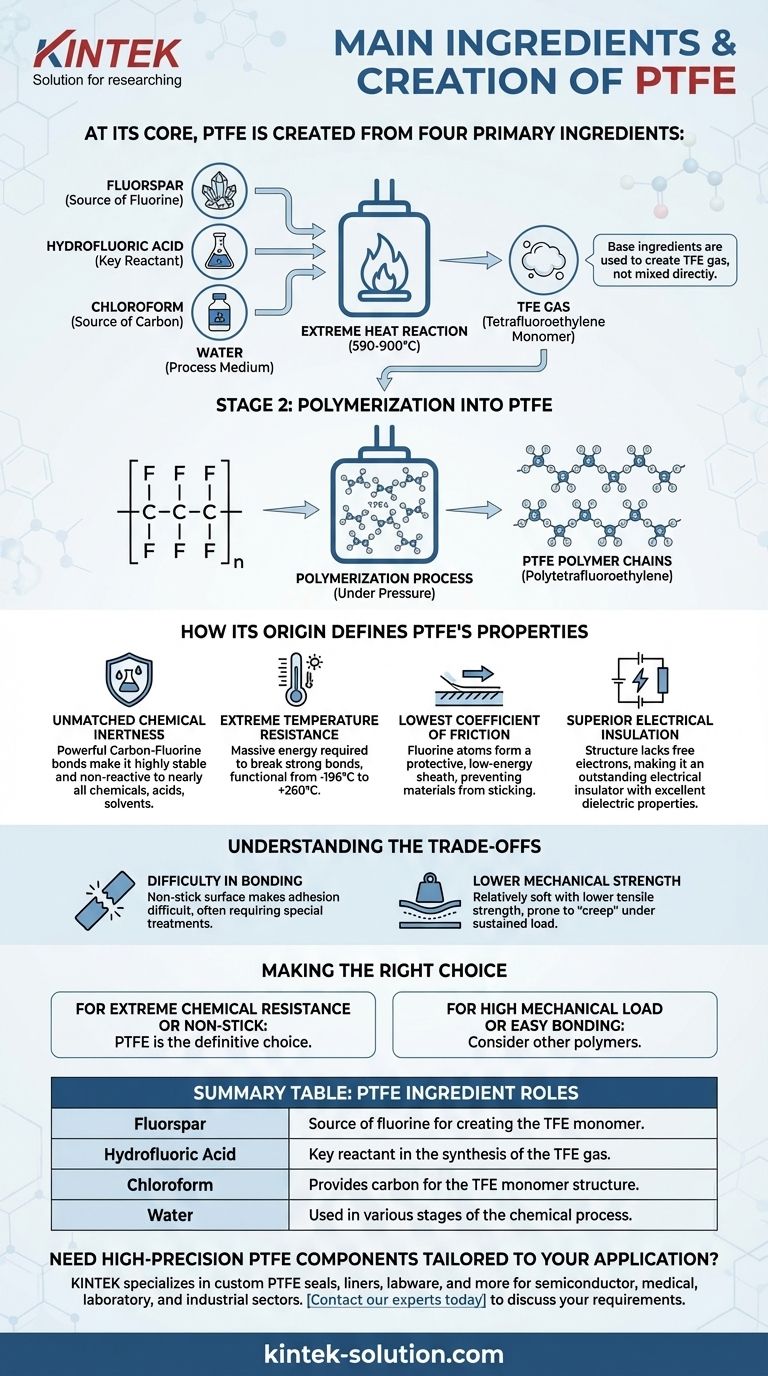
At its core, Polytetrafluoroethylene (PTFE) is created from four primary ingredients. These are fluorspar, hydrofluoric acid, chloroform, and water. These components are subjected to extreme heat in a chemical reaction chamber, which synthesizes the monomer gas that ultimately forms the stable polymer we know as PTFE.
The essential thing to understand is that the base ingredients are not mixed directly to form the final plastic. Instead, they are used to create a gas called tetrafluoroethylene (TFE), which then undergoes a process called polymerization to become PTFE.

From Raw Materials to a Unique Polymer
The creation of PTFE is best understood as a two-stage chemical process. The first stage creates the fundamental building block, and the second assembles those blocks into the final material.
Stage 1: Synthesizing the TFE Monomer
The initial ingredients—fluorspar, hydrofluoric acid, and chloroform—are the raw materials for a chemical synthesis process.
When heated to temperatures between 1094-1652°F (590-900°C), these components react to produce tetrafluoroethylene (TFE), a colorless and odorless gas. This gas is the "monomer," the single molecular unit that will be linked together.
Stage 2: Polymerization into PTFE
Once the TFE gas is created and purified, it undergoes polymerization.
In this step, individual TFE molecules are linked together under pressure to form long, stable molecular chains. The result of this process is the solid polymer, PTFE.
How Its Origin Defines PTFE's Properties
The simple, incredibly strong chemical structure born from this process is directly responsible for PTFE's legendary characteristics. The bond between carbon and fluorine atoms is one of the strongest in organic chemistry.
Unmatched Chemical Inertness
The powerful Carbon-Fluorine bonds create a highly stable, non-reactive material. This is why PTFE is resistant to nearly all industrial chemicals, acids, and solvents.
Extreme Temperature Resistance
These same strong bonds require a massive amount of energy to break. This gives PTFE its exceptionally wide operating temperature range, remaining functional from cryogenic levels (-196°C) up to +260°C.
The Lowest Coefficient of Friction
The fluorine atoms form a protective, low-energy "sheath" around the polymer's carbon backbone. This smooth, non-reactive surface prevents other materials from sticking to it, giving PTFE the lowest friction of any known solid.
Superior Electrical Insulation
The structure of the PTFE molecule does not have any free electrons that can move when a voltage is applied. This makes it an outstanding electrical insulator and gives it excellent dielectric properties.
Understanding the Trade-offs
While its properties are remarkable, the chemical nature of PTFE also creates inherent limitations that are critical to understand.
Difficulty in Bonding
The same non-stick, low-energy surface that makes PTFE so valuable also makes it extremely difficult to bond to other materials. Special surface treatments are often required to glue or adhere PTFE effectively.
Lower Mechanical Strength
Compared to many other engineering plastics, PTFE is relatively soft and has lower tensile strength and abrasion resistance. It can be prone to "creep," or deforming under a sustained load.
Making the Right Choice for Your Application
Understanding PTFE's fundamental composition helps you leverage its strengths and anticipate its weaknesses.
- If your primary focus is extreme chemical resistance or a non-stick surface: PTFE is the definitive choice due to the inherent stability of its Carbon-Fluorine bonds.
- If your primary focus is high mechanical load-bearing or easy bonding: You may need to consider other polymers, as PTFE's molecular structure makes it less suitable for these demands.
Ultimately, the unique way PTFE is built from its core ingredients is what makes it an indispensable material for specialized engineering challenges.
Summary Table:
| PTFE Ingredient | Role in Synthesis |
|---|---|
| Fluorspar | Source of fluorine for creating the TFE monomer. |
| Hydrofluoric Acid | Key reactant in the synthesis of the TFE gas. |
| Chloroform | Provides carbon for the TFE monomer structure. |
| Water | Used in various stages of the chemical process. |
Need high-precision PTFE components tailored to your application?
The unique chemistry of PTFE makes it ideal for demanding environments. At KINTEK, we specialize in manufacturing custom PTFE seals, liners, labware, and more for the semiconductor, medical, laboratory, and industrial sectors. Whether you need prototypes or high-volume orders, our expertise ensures your components leverage PTFE's full potential.
Contact our experts today to discuss your specific requirements and get a quote!
Visual Guide
Related Products
- Custom PTFE Square Trays for Industrial and Laboratory Use
- Custom PTFE Teflon Balls for Advanced Industrial Applications
- Custom PTFE Parts Manufacturer for Teflon Parts and PTFE Tweezers
- Custom PTFE Sealing Tapes for Industrial and High Tech Applications
- Custom PTFE Bottles for Diverse Industrial Applications
People Also Ask
- What are the additional properties of PTFE? Beyond Non-Stick: Extreme Chemical, Thermal & Electrical Performance
- What environmental resistances does PTFE offer? Unmatched Durability for Harsh Conditions
- What are the similarities between PTFE and RPTFE? Unlocking the Core Fluoropolymer Identity
- What is PTFE and what class of plastics does it belong to? A Guide to High-Performance Fluoropolymers
- How does PTFE react to common solvents? Discover Its Near-Total Chemical Immunity



















